Answer:
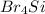 is written incorrectly
is written incorrectly
Step-by-step explanation:
In a molecular formula, the central atom in it's lewis structure is written first followed by other bonded atoms.
The least electronegative atom (except H) is the central atom in a lewis structure.
In
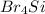 , Si is the least electronegative atom as Br has higher electronegativity than Si. Therefore Si is the central atom in lewis structure of
, Si is the least electronegative atom as Br has higher electronegativity than Si. Therefore Si is the central atom in lewis structure of
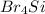 . So molecular formula of
. So molecular formula of
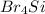 should be written as
should be written as
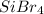 .
.
In all other cases, the least electronegative atom have been written first.
So,
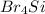 is written incorrectly.
is written incorrectly.