Hello!
The answer is:
Hence, the new pressure will be 2.07 atm.
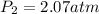
Why?
Since we know that the gas is inside of a rigid container, meaning that the volume will be kept constant, we can solve the problem using the Gay-Lussac's Law.
The the Gay-Lussac's Law establishes that when an ideal gas is kept at constant volume, the pressure and the temperature will be proportional.
We need to pay special attention when we are working with the Gay-Lussac's Law since its equations with absolute temperatures (Kelvin ), so, if we are working with relative temperatures such as Celsius degrees or Fahrenheit degrees, we need to convert the temperatures to Kelvin (absolute temperature)
We can convert from Celsius degrees to Kelvin using the following formula:
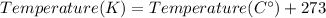
Then we have the Gay-Lussac's equation:
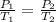
Therefore, we are given the following information:
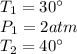
Therefore, converting the given temperatures from Celsius degrees to Kelvin, we have:
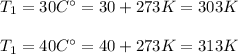
Now, calculating we have:
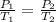
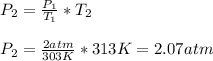
Hence, the new pressure will be 2.07 atm.
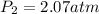
Have a nice day!