Answer: The correct answer is Option B.
Step-by-step explanation:
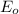 values are known as standard reduction potential values. More the positive reduction potential values, the metal will easily gain electron and will undergo reduction reaction easily.
values are known as standard reduction potential values. More the positive reduction potential values, the metal will easily gain electron and will undergo reduction reaction easily.
The standard reduction potential values of the given metals are:

Copper has positive reduction potential than lead and thus, will undergo reduction reaction easily. It will gain electrons from other substance. So, the other substance will easily loose electrons and it will therefore oxidize.
Hence, the correct answer is Option B.