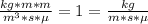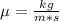Gasoline is predominantly octane, C8H18. Something like soap would be a great homogenizer. Soap is composed of a long hydrocarbon chain with a tiny, highly polar tip on one end. Usually, the soap is the anion of a salt, NaX. This allows the polar end of the soap to stick to water, while the nonpolar end sticks to the oil.