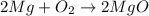Answer: d.) The same atoms are present both before and after the reaction
Explanation:-
A chemical reaction is defined as a reaction in which a change in chemical composition takes place. A new substance is formed in these reactions.
Example: Oxidation of magnesium
According to the law of conservation of mass, mass can neither be created nor be destroyed. Thus the mass of products has to be equal to the mass of reactants. The number of atoms of each element has to be same on reactant and product side. Thus chemical equations are balanced.
The balanced reaction for oxidation of magnesium is: