Answer:
The pressure correction in the ideal gas equation accounts for the intermolecular attractive forces between gas molecules. Vanderwaal constant 'a' measures the magnitude of intermolecular attractive forces between the particles.
Thevolume correction to the total volume per mole occupied by gas molecules, it closely corresponds to the volume per mole of the liquid state, whose molecules are closely layered. Vanderwaal constant 'b' measures the volume excluded by a mole of particles.
Vander waals equation followed by real gas is:
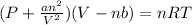
where,
P = pressure of gas
V = volume of gas
n = number of moles of gas
R = gas constant
T = temperature of gas