Answer: The equation is given below.
Step-by-step explanation:
When nitric oxide reacts with hydrogen gas to form ammonia gas and water vapor, the equation for the following reaction follows:
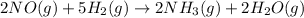
Reactants of the reaction are nitric oxide and hydrogen gas and all are present in gaseous phase.
Products of the reaction are ammonia and water vapor and all are present in gaseous phase.