Answer : The mass of calcium chloride is, 116.84 grams
Solution : Given,
Molar mass of calcium chloride,
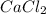 = 110.98 g/mole
= 110.98 g/mole
Number of molecules of calcium chloride =
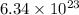
As we know that,
1 mole of calcium chloride contains
 molecules of calcium chloride
molecules of calcium chloride
or,
1 mole of calcium chloride contains 110.98 grams of calcium chloride
Or, we can say that
As,
 molecules of calcium chloride present in 110.98 grams of calcium chloride
molecules of calcium chloride present in 110.98 grams of calcium chloride
So,
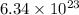 molecules of calcium chloride present in
molecules of calcium chloride present in
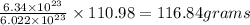 of calcium chloride
of calcium chloride
Therefore, the mass of calcium chloride is, 116.84 grams