Step-by-step explanation:
In a displacement reaction, more reactive element displaces less reactive element. Whereas in a double displacement reaction there is exchange of ions of two compounds.
For example,
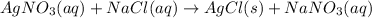
This is a double displacement reaction as ions of both compounds
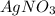 and NaCl are exchanged by each other.
and NaCl are exchanged by each other.