Answer:
Step-by-step explanation:
Chemical formula of any compound is represented by symbols of each element present in the compound along with number of atoms of each element. Number of each element is represented by subscript.
Cation is written first and anion is written after that.
Given compound = Sulfur tetrafluoride
Chemical symbol of sulfur = S
Chemical symbol of fluorine = F
In the given compound, one atom of sulfur and four atoms of fluorine is present.
In the given compound, S is cation having oxidation state +4 and anion is present as anion having oxidation state -1.
So, the chemical formula of Sulfur tetrafluoride is
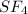
So, option d is correct.