Answer: Option (A) is the correct answer.
Step-by-step explanation:
According to Aufbau's principle, in the ground state of an element, electrons first enter into the lowest energy level before entering into higher energy level.
This means that an "s" sub-shell has lower energy than a "p" sub-shell so, s shell will fill first and then p shell will be filled.
For example, atomic number of carbon atom is 6 and its electronic distribution is
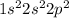 .
.
Thus, we can conclude that an electron in the first energy level of the electron cloud has a lower energy than an electron in the third energy level.