Answer:- Third choice is correct, 17.6 moles
Solution:- The given balanced equation is:
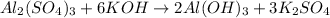
We are asked to calculate the moles of potassium hydroxide needed to completely react with 2.94 moles of aluminium sulfate.
From the balanced equation, there is 1:6 mol ratio between aluminium sulfate and potassium hydroxide.
It is a simple mole to mole conversion problem. We solve it using dimensional set up as:
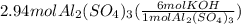
= 17.6 mol KOH
So, Third choice is correct, 17.6 moles of potassium hydroxide are required to react with 2.94 moles of aluminium sulfate.