The rate of reaction between oxygen and copper is higher at high temperature. The rate of reaction between oxygen and copper is lower at room temperature.
at high temperature copper penny will react with oxygen as shown below
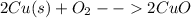
The reaction does not occur easily at room temperature
We can say that the reaction between copper and oxygen is an endothermic reaction so the rate of reaction is high at high temperature.
The bond energy of oxygen molecules need energy to react with copper and form copper oxide.