Answer:The correct answer is option C.
Explanation;
Water has unique property that is, it expands on freezing.Which is the reason for less density of ice than water. The cracking of the bottle was due to the expansion of water (present inside the bottle) on freezing.
Generally, density is inversely proportional to the volume of the substance.

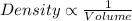
As we know ,that water expands on freezing which means that volume increases. And with increase in volume of density decreases.
Hence, the correct answer is option C.