Given:
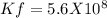
The reaction is

We know that
Δ
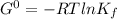
Δ
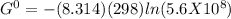
Δ
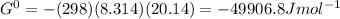
For the given reaction the reaction quotient will be :
Q = \frac{[Ni(NH_{3} )_{6} ^{+2}] }{[Ni^{+2} ][NH_{3}]^{6}
On putting values
Q will be equal to =
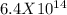
The relation between standard and other free energy change is
ΔG = Δ
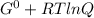
Thus
ΔG = (-49906.8) + (8.314)(298) ( ln (
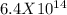 )
)
ΔG = (-49906.8) + 84466
ΔG = 34535 J / mol K
ΔG is positive so reaction is not spontaneous in forward direction
It will proceed in reverse direction, it will move from product to reactant