Answer:
lowering the temperature of the reaction
Step-by-step explanation:
According to Le Chatelier's principle, when a system at equilibrium is subject to changes in temperature, pressure or concentration then the equilibrium will shift in a direction so as to undo the effect of the induced change.
The given reaction is:
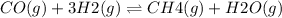
ΔH = -206 kJ/mol.
Since the enthalpy change is negative, heat is evolved and the reaction is exothermic. Therefore, if the temperature of the reaction is lowered then it will shift the equilibrium in a direction of higher temperature i.e. towards evolution of heat. This will shift it to the right.