Answer:
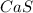
Step-by-step explanation:
Hello!
In this case, since calcium's oxidation state when forming ionic bonds is +2 and sulfur's oxidation state when bonding those bonds is -2, for the required formula we write:
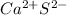
Now, since they have the same charge number, we infer the ionic compound formed when they bond is calcium sulfide:
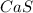
Best regards!