The new temperature : 11.56 °C
Further explanation
Boyle's law and Gay Lussac's law
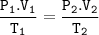
P1 = initial gas pressure (N/m² or Pa)
V1 = initial gas volume (m³)
P2 = final gas pressure
V2 = final gas volume
T1 = initial gas temperature (K)
T2 = final gas temperature
V₁=4.39 L
T₁=44+273=317 K
P₁ = 729 torr = 0,959211 atm
V₂=3.78 L
P₂= 1 atm
