Answer:

Step-by-step explanation:
First, let's find the density of the gold bracelet.
Density can be found by dividing the mass by the volume.
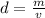
The mass of the bracelet is 412 grams and the volume is 22.4 milliliters.
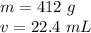
Substitute the values into the formula.
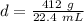
Divide.
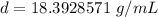
Let's round to the nearest hundredth.
- The 2 in the thousandth place tells us to leave the 9 in the hundredth place.
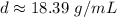
The density of the bracelet is about 18.39 grams per milliliter.
Now, let's compare the bracelet's density to pure gold's density.
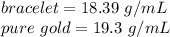
It's quite clear that the two values are not equal. Therefore, the bracelet cannot be made of pure gold, because it's density doesn't match pure gold's.