Answer:
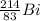
Step-by-step explanation:
In beta emission, a neutron is converted into a proton thereby reducing the neutron:proton (N/P) ratio.
When a beta emission occurs, the mass number remains the same while the nuclear charge increases by one unit. The daughter nucleus is found one place to the right of its parent in the periodic table.