Answer:
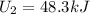
Step-by-step explanation:
Hello!
In this case, since the fist law of thermodynamics helps us to understand how the change in internal energy is defined in terms of the head added to the system and the work done by the system:
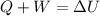
In such a way, since 8.3 kJ of heat are absorbed by the system, 3.5 kJ are done by the system and the initial internal energy is 43.5 kJ, the final internal energy turns out:
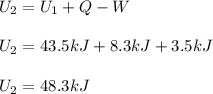
Best regards!