C. the square root of the mass of the particles.
Further explanation
Graham's law: the rate of effusion of a gas is inversely proportional to the square root of its molar masses or
the effusion rates of two gases = the square root of the inverse of their molar masses:
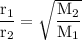
or
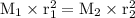
From this equation shows that the greater the mass of the gas, the smaller the effusion rate of the gas and vice versa, the smaller the mass of the gas, the greater the effusion velocity.
So if both gases are at the same temperature and pressure, the above formula can apply