Answer:
i. F = 1.3 x
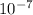 N
N
ii. The direction of the force of attraction exerted by the proton on the electron is towards the itself (i.e a pull).
Step-by-step explanation:
Since the given charges are opposite, then the force of attraction is experienced. The force of attraction between the two charges can be determined by:
F =
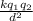
where F is the force, k is the constant,
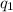 is the charge of the electron,
is the charge of the electron,
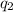 is the charge on the proton, and d is the distance between them.
is the charge on the proton, and d is the distance between them.
So that; k = 9.0 x
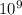 N
N
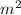
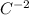 ,
,
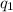 = 1.6 x
= 1.6 x
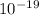 C,
C,
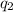 = 1.6 x
= 1.6 x
Thus,
F =
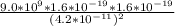
=
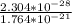
= 1.3061 x
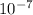
F = 1.3 x
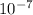 N
N
The force between the charges is 1.3 x
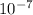 N.
N.
ii. The direction of the force of attraction exerted by the proton on the electron is towards the itself.