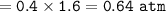The partial pressure of N₂ : A. 0.64 atm
Further explanation
Dalton's law of partial pressures states that the total pressure of a mixture of gases is equal to the sum of the partial pressures of the component gases
Can be formulated:
P tot = P1 + P2 + P3 ....
The partial pressure is the pressure of each gas in a mixture
For partial gas :
Pgas₁=x₁.P tot
x₁ = mole fraction of gas 1
mol fraction CH₄=0.6
P tot = 1.6 atm
mol fraction N₂ :

The partial pressure of N₂ :