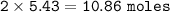10.86 moles of water are produced
Further explanation
A reaction coefficient is a number in the chemical formula of a substance involved in the reaction equation. The reaction coefficient is useful for equalizing reagents and products.
Reaction
Pb + PbO₂ + 2H₂SO₄ ⇒ 2PbSO₄ + 2H₂O ( a car battery reaction)
From the equation :
mol ratio PbO₂ : H₂O = 1 : 2⇒ 1 mol PbO₂ produces 2 mol H₂O
so for 5.43 mol PbO₂ consumed, mol H₂O :