Given :
A seawater sample contains 2.7 mg chlorophyll m⁻³.
To Find :
What is the chlorophyll concentration in units of mg L⁻¹.
Solution :
We know, 1 m³ = 1000 L .
So, 1 m⁻³ = 10⁻³ L⁻¹
Concentration is :
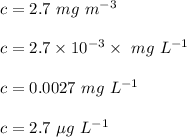
Therefore, the chlorophyll concentration in units of mg L⁻¹ is
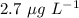 .
.
Hence, this is the required solution.