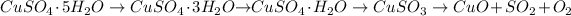Answer:
Blue vitriol is crystal copper sulphate
Step-by-step explanation:
The black oxide is the higher oxide of Copper i.e Cu. It is also called as tenorite. It is solid black in color. It is formed by heating copper with air.
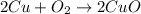
Blue vitriol is the crystalline form of copper sulphate. It is also known as copper (II) sulphate.
When heating, the blue vitriol undergoes various steps of changes to the black oxide of copper.
The chemical reaction is --