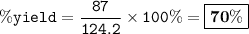Percent yield = 70%
Further eplanation
Percent yield is the comparison of the amount of product obtained from a reaction with the amount you calculated
General formula:
Percent yield = (Actual yield / theoretical yield )x 100%
An actual yield is the amount of product actually produced by the reaction. A theoretical yield is the amount of product that you calculate from the reaction equation according to the product and reactant coefficients
Reaction
2H₂ (g) + O₂ (g) → 2H₂O (g)
- mass of H₂O (theoretical) :