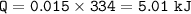1. Q=112.8 kJ
2. Q=5.01 kJ
Further explanation
The heat required for phase change :
Q = mLf
Lf=latent heat of fusion
- vaporization/condensation
Q = mLv
Lv=latent heat of vaporization
1.
m=50 g=0.05 kg
Lv (water) = 2256 kJ/kg
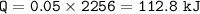
2.
m=15 g=0.015 kg
Lf for water = 334 kj/kg