Answer:
3.4g
Step-by-step explanation:
Given parameters:
Mass of ethanol = 2.911g
Unknown:
Mass of water vapor = ?
Solution:
To solve this problem, let us write the balanced reaction equation first;
C₂H₅OH + 3O₂ → 2CO₂ + 3H₂O
Now find the number of moles of ethanol;
Number of moles =

Molar mass of ethanol = 2(12) + 5(1) + 16 + 1 = 46g/mol
Number of moles =
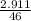 = 0.06mole
= 0.06mole
So;
1 mole of ethanol will produce 3 moles of water vapor
0.06mole of ethanol will produce 0.06 x 3 = 0.19moles of water vapor
Mass of water vapor = number of moles x molar mass
Molar mass of H₂O = 2(1) + 16 = 18g/mol
Mass of water vapor = 0.19 x 18 = 3.4g