Answer:
B. NaBr
D. KOH
Step-by-step explanation:
Below is the solubility rules given for you knowledge.
Salts of
- Group 1 elements are soluble(
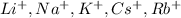 )
) - Ammonium ion is soluble (
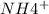 )
) - The nitrate are generally soluble(
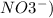
- of Cl- , Br- , and I- are soluble, except Ag+ , Pb+2, and (Hg2)+2
- most sulfate are soluble, except Ba+2, Ca+2,Pb+2, Ag+, Sr+2.
- most hydroxide salts are only slightly soluble, except NH+4, Li+, Na+, K+
- Most carbonates are insoluble (CO3 2-) Except group 1 and NH+4
- most phosphate are insoluble except group 1 and NH+4
so using the rules above
NaBr , KOH are soluble, Pb(OH)2 is slightly soluble and AgCl is not soluble.