Answer:
0.87mole
Step-by-step explanation:
The reaction equation is given as;
4Fe + 3O₂ → 2Fe₂O₃
Number of moles of Fe₂O₃ in 138.5g
To solve this problem;
Number of moles =

Molar mass of Fe₂O₃ = 2(56) + 3(16) = 160g/mol
Now;
Number of moles =
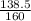 = 0.87mole
= 0.87mole