Answer:
1.44 x 10²⁵ ions of Na⁺
Step-by-step explanation:
Given parameters:
Mass of NaCl = 1.4kg = 1400g
Unknown:
Number of ions of sodium = ?
Solution:
The compound NaCl in ionic form can be written as;
NaCl → Na⁺ + Cl⁻
In 1 mole of NaCl we have 1 mole of sodium ions
Now, let us find the number of moles in NaCl;
Number of moles =

Molar mass of NaCl = 23 + 35.5 = 58.5g/mol
Number of moles =
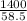 = 23.93mol
= 23.93mol
So;
Since 1 mole of NaCl gives 1 mole of Na⁺
In 23.93 mole of NaCl will give 23.93 mole of Na⁺
1 mole of a substance = 6.02 x 10²³ ions of a substance
23.93 mole of a substance = 6.02 x 10²³ x 23.93
= 1.44 x 10²⁵ ions of Na⁺