Answer:

Step-by-step explanation:
Density can be found by dividing the mass by the volume.
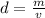
The mass of the copper is 89.6 grams.
The volume is 10 cubic centimeters.
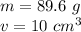
Substitute the values into the formula.
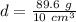
Divide.
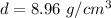
The density of copper is 8.96 grams per cubic centimeter.