The Concept:
We are given the density of a sample of the metal = 11.4 grams / cm³
and we need to find the volume occupied by a sample of 30.5 grams
For this solution, we will use dimensional analysis
from the given information, we can also say that the density of the metal is:
1 cm³ / 11.4 grams
If we multiply this value by 30.5 grams, the 'grams' in the numerator and the denominator will cancel out and we will be left with the volume occupied by 30.5 grams of the metal
Solving for the volume:
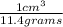 X 30.5 grams = (30.5 / 11.4) cm³
X 30.5 grams = (30.5 / 11.4) cm³
Volume of 30.5 grams of the sample = 2.68 cm³