Answer:
Density,
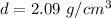
Step-by-step explanation:
Given that,
Mass of a flask is 450 g
Volume of benzene added to the flask is 145 mL or 145 cm³
The weight of the flask and benzene is found to be 754 g.
We need to find the density of the benzene.
Weight of benzene added = total weight of flask and benzene-mass of flask
m = 754 g - 450 g
m = 304 g
Density = mass/volume
So,
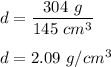
So, the density of the benzene is
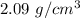 .
.