Answer: 0.287 M
Step-by-step explanation:
Molarity is
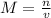 . n is moles or solute and v is volume of solution in liters. Since we are given the grams of potassium iodide, we want to convert that into moles. With the volume in mL, we want to convert that to liters.
. n is moles or solute and v is volume of solution in liters. Since we are given the grams of potassium iodide, we want to convert that into moles. With the volume in mL, we want to convert that to liters.
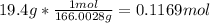
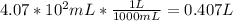
Now that we have moles and liters, we can find molarity.
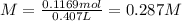
Now, we know the molarity is 0.287 M.