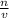Answer:
Molarity and normality describe the numbers (moles) of reactants or products dissolved in one liter of solution. Molarity: M = moles of solute contained in one liter of solution. ... Normality is always a multiple of molarity. It describes the “equivalent” moles of reactants involved in chemical reactions.
Step-by-step explanation:
Normality is a measure of concentration equal to the gram equivalent weight per litre of solution. Gram equivalent weight is the measure of the reactive capacity of a molecule. The solute's role in the reaction determines the solution's normality. Normality is also known as the equivalent concentration of a solution.
Molar concentration is a measure of the concentration of a chemical species, in particular of a solute in a solution, in terms of amount of substance per unit volume of solution.
Formula for Molar: M =