Answer:
The answer is 2.91 g/mL
Step-by-step explanation:
The density of a substance can be found by using the formula
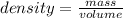
volume = final volume of water - initial volume of water
volume = 30 - 23 = 7 mL
We have

We have the final answer as
2.91 g/mL
Hope this helps you