Answer:
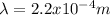
Step-by-step explanation:
Hello!
In this case, since the relationship between energy and wavelength is defined via the speed of light and the Planck's constant as shown below:
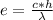
Since c stands for the speed of light and h for the so-called Planck's constant, we can compute the wavelength as follows:
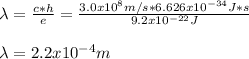
Best regards!