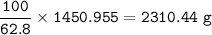The percentage yield of the reaction : 93.3%
Mass of FeCO₃ 2310.44 g
Further explanation
The reaction equation is the chemical formula of reagents and product substances
A reaction coefficient is a number in the chemical formula of a substance involved in the reaction equation. The reaction coefficient is useful for equalizing reagents and products
Reaction
4 FeCO₃ + O₂ ⇒ 2 Fe₂O₃ + 4CO₂
MW FeCO₃ : 115,854 g/mol
MW Fe₂O₃ : 159,69 g/mol
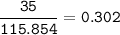
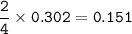
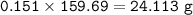
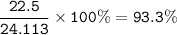
mol of 1 kg Fe₂O₃ = 1000 g
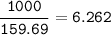
mol of FeCO₃
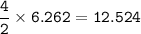
mass of FeCO₃
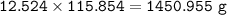
a purity of 62.8%