Answer:

Step-by-step explanation:
Hello.
In this case, since percent compositions are computed by knowing the number of atoms of the required atom, its atomic mass and the molar mass of the compound at which it is, thus, for calcium we have:
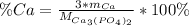
Thus, since calcium weights 40.08 g/mol and calcium phosphate 310.2 g/mol, the by mass percent of calcium turns out:
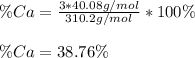
Best regards!