Answer:
0.019122 grams of CuS(s) would be produced .
Step-by-step explanation:
Given , volume of
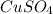 is 4ml = 0.004 L
is 4ml = 0.004 L
volume of
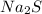 is 2ml = 0.002 L
is 2ml = 0.002 L
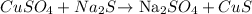
Moles of
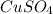 = Molarity x volume (L)
= Molarity x volume (L)
= 0.1M x 0.004 L
= 0.0004 moles
Moles of
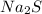 = 0.1M x 0.002 L
= 0.1M x 0.002 L
= 0.0002 moles
1 mole of
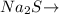 1 mole of
1 mole of
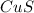
Therefore , 0.0002 moles
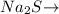 1mole of
1mole of
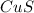
Mass of
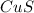 = Moles x molar mass
= Moles x molar mass
= 0.0002 x 95.611
= 0.019122 grams .
Hence the answer is 0.019122 grams.