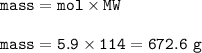Mass =672.6 g
Further explanation
The mole is the number of particles contained in a substance
1 mol = 6.02.10²³
Moles can also be determined from the amount of substance mass and its molar mass
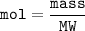
MW = molecular weight
MW C₈H₁₈ = 8x12 + 18 x1 = 114 g/mol
mass: