Answer:
690 g IrI₃
Step-by-step explanation:
To convert from moles to grams, you have to use the molar mass of the compound. The molar mass of IrI₃ is 572.92 g/mol. You use this as the unit converter in this equation:
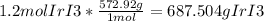
Round to the lowest number of significant figures which is 2 to get 690 g IrI₃.