Answer:
The equilibrium constant change. For an endothermic reaction, heat is a reactant. An increase in temperature and heat favors the forward reaction and the value of Kc increases.
Step-by-step explanation:
Hello.
In this case, regarding reactions in equilibrium in which heat is a reactant as those exemplified by:
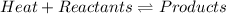
We infer that the heat of reaction is positive since the reactants have more energy in their ground state than the products making them endothermic. Moreover, since the Le' Chatelier's principle states that increasing the reaction temperature in endothermic reactions, the forward reaction (towards products) is favored because endothermic reactions absorb heat in the form temperature raise, the required statement is:
The equilibrium constant change. For an endothermic reaction, heat is a reactant. An increase in temperature and heat favors the forward reaction and the value of Kc increases.
Regards.