Given parameters:
Number of moles of Nitrogen = 2.0mole
Temperature of the gas = 1000c
Pressure on the gas = 100000Pa
Gas constant R = 8.31J/molK = 0.082atmdm³mol⁻¹k⁻¹
Unknown:
Volume in cubic meter and cubic decimeter = ?
Solution:
The ideal gas law is given as;
PV = nRT
where P is the pressure on the gas
V is the volume of the gas
n is the number of moles
R is the gas constant
T is the temperature of the gas
Let us convert to appropriate units;
1000°C to K;
K = 1000 + 273 = 1273K
100000Pa to atm;
101325Pa = 1atm
100000Pa = 0.99atm
Input the parameters and solve for the unknown;
0.99 x V = 2 x 0.082 x 1273
V = 210.9dm³
to m³;
1000dm³ = 1m³
210.9dm³ =
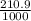 = 0.21m³
= 0.21m³