Answer:
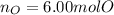
Step-by-step explanation:
Hello.
In this case, since the molecular formula of barium sulfate is:
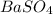
Which has four oxygen atoms, we can also say that one mole of barium sulfate has four moles of oxygen; in such a way, the moles of oxygen atoms in 1.50 moles of barium sulfate are:
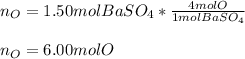
Best regards.