Answer:

Step-by-step explanation:
Hello.
In this case, when chlorine gets ionized by gaining one electron it goes:

Whose electron configuration is the same as argon:
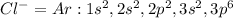
Since the both of them have 18 electrons, they become isoelectric with the same neutral charge.
Best regards.