Answer:
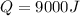
Step-by-step explanation:
Hello.
In this case, since the energy involved in a heating process is computed via:
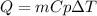
Whereas m is the mass (0.4 kg), Cp is the specific heat (900 J/kg•°C) and ΔT the temperature rise (25 °C), the required heat turns out:
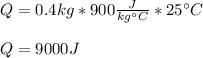
It means that 9000 J of energy is transferred to attain that temperature raise.
Regards.